Stealth Nucleosides
DNA Stealth Nucleosides :
DNA Stealth NucleosidesTM are incorporated during viral replication into the viral genome by reverse transcriptase (RNA to DNA). DNA Stealth Nucleosides can target HIV and hepatitis B virus (HBV). In contrast to currently available DNA nucleoside chain terminators, which ultimately lose their effectiveness because of the emergence of viral resistance, nucleoside analogs developed for SVM work with the very mechanism, lack of fidelity during replication, leading to the emergence of viral resistance, hence their "stealth."
Novel approaches to antiretroviral therapy are required with a low likelihood of viral resistance. In infected individuals, HIV mutates at a rate approximately a million times greater than that of cellular DNA genomes. Most of these mutations are probably lethal or crippling, which explains why most viral particles are not infectious, but rare mutations may allow the virus to survive in the presence of an antiviral. Selective viral mutagenesis (SVM) relies on the very same lack of fidelity of HIV reverse transcriptase (RT), which is responsible for thwarting current therapeutics. The purpose of DNA Stealth Nucleosides (DSN) that work by SVM is to increase the mutation rate of HIV to the point of non-viability with minimal toxicity to host cells. This is accomplished by the use of DNA analogs incorporated by HIV RT into the viral genome. Mutations are incurred upon misincorporation during subsequent DNA directed DNA polymerization of the viral genome (DNA:DNA duplex formation). The efficient incorporation of DSNs by RT, the high rate of HIV replication, the lack of repair of a DSN incorporated in a RNA:DNA hybrid produced by RT, the low affinity of DSNs for human polymerases and efficient repair of a DSN should it be incorporated in a cellular DNA:DNA duplex are major factors in the preferential accumulation of mutations in HIV.
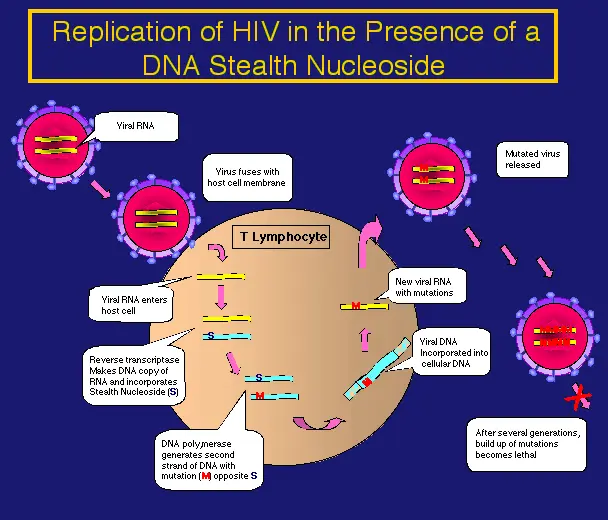
It has been demonstrated through studies of the crystal structure of HIV RT that one reason for resistance to current nucleoside analog chain terminators is the lack of 3'-OH group allowing chain elongation on the deoxyribose moiety, explaining why cross resistance is common. In contrast to current nucleoside analog RT chain terminators, DSNs have a 3’-OH group and are incorporated into the growing DNA chain by reverse transcriptase. Presence of a 3’-OH group has also been demonstrated to enhance the efficiency of repair should a nucleoside analog be incorporated into DNA.
RNA Stealth Nucleosides :
RNA Stealth NucleosidesTM have a broad spectrum of antiviral activity, including RNA viruses, HIV and HBV. For classical RNA viruses, RNA Stealth Nucleosides are incorporated by viral RNA polymerase (RNA to RNA). Koronis is particularly interested in treating hepatitis C virus (HCV) with this class of nucleoside. In the case of HIV, RNA Stealth Nucleosides are incorporated into the viral genome at the level of transcription (DNA to RNA) and have a similar mode of action. It is possible to envision treating patients co-infected with HIV and HCV with a single RNA Stealth Nucleoside.
Nucleoside analogs have been used extensively for the treatment of infections by DNA viruses and retroviruses. These analogs have been designed to be incorporated into DNA by DNA polymerases or reverse transcriptases. Once incorporated they cannot be further extended and thus terminate DNA synthesis. There is immediate selective pressure for the development of resistance against such analogs resulting from mutations in polymerase that prevent incorporation of the nucleoside analog
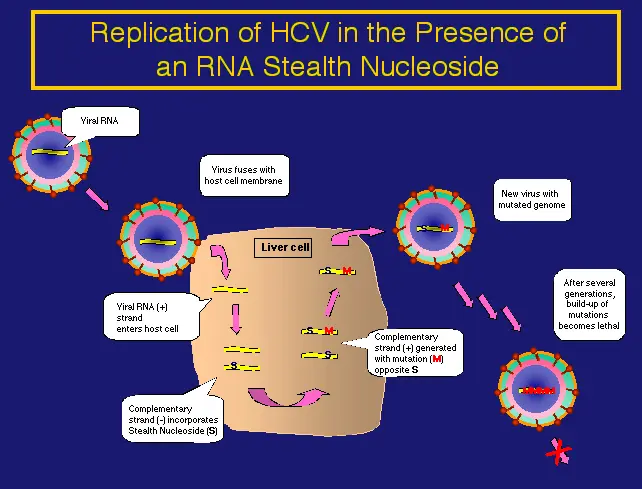
Riboviruses have RNA instead of DNA genomes and are the most common viruses. Ribavirin is currently the only broad-spectrum therapeutic approved for the treatment of riboviruses. For the treatment of these viruses, Koronis Pharmaceuticals proposes an entirely different approach to therapy, one that is not thwarted by the high rate of mutation of riboviruses. As with HIV, the rate of mutation of riboviruses is so large, and ribovirus genomes are so informationally dense, that even a modest increase the rate has been modeled to extinguish the population. Koronis Pharmaceuticals has been testing RNA Stealth Nucleoside (RSN) that miscode at high frequency and are preferentially incorporated into viral RNAs. With each cycle of viral infection, there ensues a chain like increase in the number of mutations in the viral genome. Eventually the number of mutations in each viral genome is so large that no active virally encoded proteins are produced. This approach provides a unique method to exceed the error threshold for viral viability without causing significant toxicity to host cells.