Nucleosides: Mechanisms and Innovations
Understanding Nucleosides in Viral Research
Nucleosides are molecules made of a base (adenine, guanine, cytosine, uracil, or thymine) attached to a sugar (ribose in RNA or deoxyribose in DNA). In antiviral research, nucleoside analogs often have modified sugars but natural bases. Another approach uses natural sugars with modified bases to affect viral replication.
For example, nucleoside analog reverse transcriptase inhibitors (NRTIs) for HIV have modified sugars that lack a 3’-OH group. This stops the viral DNA from extending during replication, a process called "chain termination."
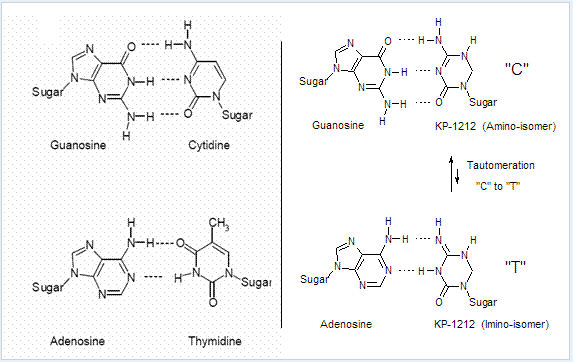
Using natural sugars in nucleosides can help them be recognized by viral enzymes, allowing them to be added to viral genomes. Changes in the bases can cause errors (mutations) in the virus, like guanine-to-adenine or cytosine-to-thymine substitutions, which disrupt replication.
Experimental Compounds in Viral Research
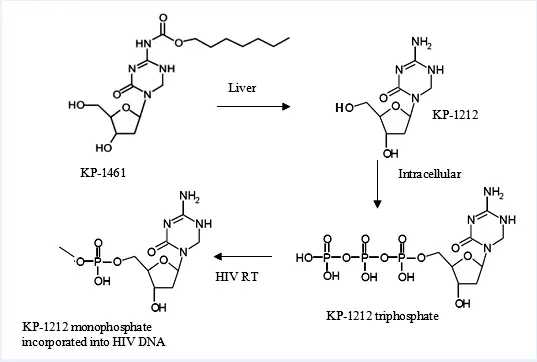
Research on compounds like KP-1212 shows they can enter viral genomes and cause mutations that interfere with replication. These compounds can form different base pairs, which makes them effective against viruses. Studies on KP-1212 in the lab showed it caused mutations in HIV without harming normal cells.
KP-1461, a version of KP-1212 that can be taken as a pill, has been tested in early clinical trials. The trials looked at its safety and ability to work against drug-resistant HIV. Results showed that it was well tolerated at all doses tested.
Further studies are being done to explore how nucleoside analogs can help fight viral infections and improve treatment options.